결과로 입증한



대한민국 NO.1 양평해장국
연간 판매량으로 검증한 해장국 대표 맛집.
Serving
72,760,704
-








어은터널 1호점
Since 2001
22년 운영으로 검증한, 오래된 노포의 안정성
Since 2001
-





익산부O점 120평 연 누적 매출
18억 8,409만원
놀라운 평당 매출로 검증한 성공 창업 아이템
18억 8,409만원
-








우가양평해장국
대한민국이 함께할 국민 브랜드
가장 많은 양평해장국으로 가장 많은 단골 손님들과 함께한
20년지기 국민 브랜드 우가양평해장국대한민국이 함께할 국민 브랜드
모든 가맹점의
 을 만들다
을 만들다
소형 매장부터 중대형 매장까지
'우가양평해장국'은 모든 유형의 창업 모델에서
탄탄한 성공을 검증 했습니다.

-
30평대 소형 매장
0 , 0 0 0
-
60평대 중형 매장
0 , 0 0 0
-
90평 이상 대형 매장
0 억 0 , 0 0 0
고민할 필요 없는
상권과
상권과 입지에 따라 탄력있는 3way 운영 지원으로
매장의 크기와 관계없이 높은 매출을 완성합니다.
중화산점
9,877 만원
월 최고 매출
서천점
9,670 만원
월 최고 매출
아중점
1억5,700 만원
월 최고 매출
삼천점
9,870 만원
월 최고 매출
고창점
9,230 만원
월 최고 매출
장항점
7,480 만원
월 최고 매출
부송점
1억8,200 만원
월 최고 매출
효자점
1억2,352 만원
월 최고 매출
서산점
6,873 만원
월 최고 매출
동산점
8,245 만원
월 최고 매출
손 꼽히는
 10% 매출
10% 매출
1일
0 , 0 0 0
만원
전주 아O점 1일 최고 매출
남녀노소 불문하고
온 종일 찾는

트리플 시그니처 맛집
시작은 늘 '양평해장국'이지만
'감자탕과 곱창전골'에 또 한 번 반하는 맛집.
전문성 있는 3가지 시그니처 메뉴를 통해
목표한 1일 매출을 쉽게 완성합니다.
-

0 0 0 만원
양평해장국
1일 최고 매출
-

0 0 0 만원
감자탕
1일 최고 매출
-

0 0 0 만원
곱창 전골
1일 최고 매출
점심부터 저녁까지
하루 종일 잘 되는 운영 탄력
'탕반류와 전골류' 두 가지 카테고리의 맛집으로
점심과 저녁 1일 2번의 PEAK TIME에 많은 손님이 유입됩니다.
-
소룡점
점심 1일 최고 매출
-
효자점
저녁 1일 최고 매출







복날에 더 잘 되고
명절에 더 잘 되는 가족 맛집
온 가족이 함께 모이는 공휴일에
더 없이 강력한 매출을 완성합니다.
1.7 회전
24시 전주 삼O점 1일 최대 회전율
2.4 회전
24시 전주 삼O점 1일 최대 회전율
4.6 회전
24시 전주 삼O점 1일 최대 회전율
6.3 회전
24시 전주 삼O점 1일 최대 회전율
9.5 회전
24시 전주 삼O점 1일 최대 회전율
오직 한 곳 뿐인
특허 받은
우가양평해장국이 만든 업계 유일의 레시피는 법적으로 보호 받고 있어 누구도 흉내낼 수 없습니다.

선지가 진짜 맛있는
양평해장국
전주의 맛을 담은
명품 육수
'호불호' 없이 즐겨찾는 선지
더 진하고 더 '깊은 맛'
선지 고유의 비릿한 냄새를 잡고
어디에도 없는 부드러운 식감을 완성한
우가양평해장국만의 '선지 제조법'.
맛의 고향 전주의 요리법으로 완성해
감칠맛이 뛰어난 깊은 풍미의 명품 육수.
선지 가공법
특허 제 10-1096591호
선지 육수 제조법
특허 제 10-1059725호


느리지만

전북을 넘어 호남에 소문나고 호남을 넘어 전국에 확장되기까지 20년.
다소 느리게
걸어온
'전주 출신 브랜드'답게
조금의 맛의 차이에도 민감했고
이를 완벽히 하기 위해 본사
대부분의 인력을 가맹점에 파견하며
'확산이 아닌 내실'에 집중 했습니다.
그럼에도,
꼬리에 꼬리를
무는
손님과 직원이 창업하고
가맹점이 지인에게 추천하는 창업
'가맹 사업 마케팅'과 '영업 인력'이
단 한 번도 없었지만 '브랜드 내실 가치'가
소문나며 오직 맛과 브랜딩만으로
오늘의 위치에 섰습니다.
20년간 쌓아온
성공
500호점 이상의 가맹점이
어떠한 환경에서도 완벽히 운영될 수 있는
'프랜차이즈 시스템'을 갖추고
이제 본격적인 가맹 사업을 전개합니다.

정식 가맹 사업 론칭
한해 2,000만원 상당 의 파격적인 프로모션을 지원합니다.


High Quality


-

오너셰프가 의도한
100%의 맛간편함만이 아닌 '맛의 완성도'에 집중한
완제품 형태로 전문 주방 인력 없이
누구나 쉽게 조리가 가능합니다. -
1일 3시간의
여유를 만들다주요 재료에 관한 준비 및 손질 과정이
혁신적으로 단축하고 밑작업이 거의 없어
노동 강도가 매우 낮습니다. -

자연정가의
체계적인 교육 지원최대 7일간의 '본사 조리 교육'으로
누구나 쉽게 주방의 주인이 될 수 있습니다.
자부심 높은

편한 공정도 최선책이다

높은 만족을 만들다
강력한 인프라를 통한
합리적인 공급가
· 대량 주문을 통한 본사 파잉 파워 · 분기별 협력사 비딩 시스템 · 외주 없는 직접 가공으로 중간 마진 0%
주 3회 신선 전국 배송
내륙 지방은 물론 '제주도'까지.
대한민국이라면 어디라도 가장 신선하게
모든 물류를 공급합니다.


까다롭게 완성한 생산 센터
2번의 신축과 1번의 확장
2007년 10월
· 200평 규모 생산 센터 준공2013년 10월
· 500평 규모 신 생산 센터 준공2017년 10월
· 신 생산센터 증축 준공
완벽한 시스템이 완벽한 브랜딩의 초석입니다.
모든 가맹점과 함께 오랫동안 사랑받은
브랜드가 되기 위해 시스템 개발에
많은 것을 투자합니다.

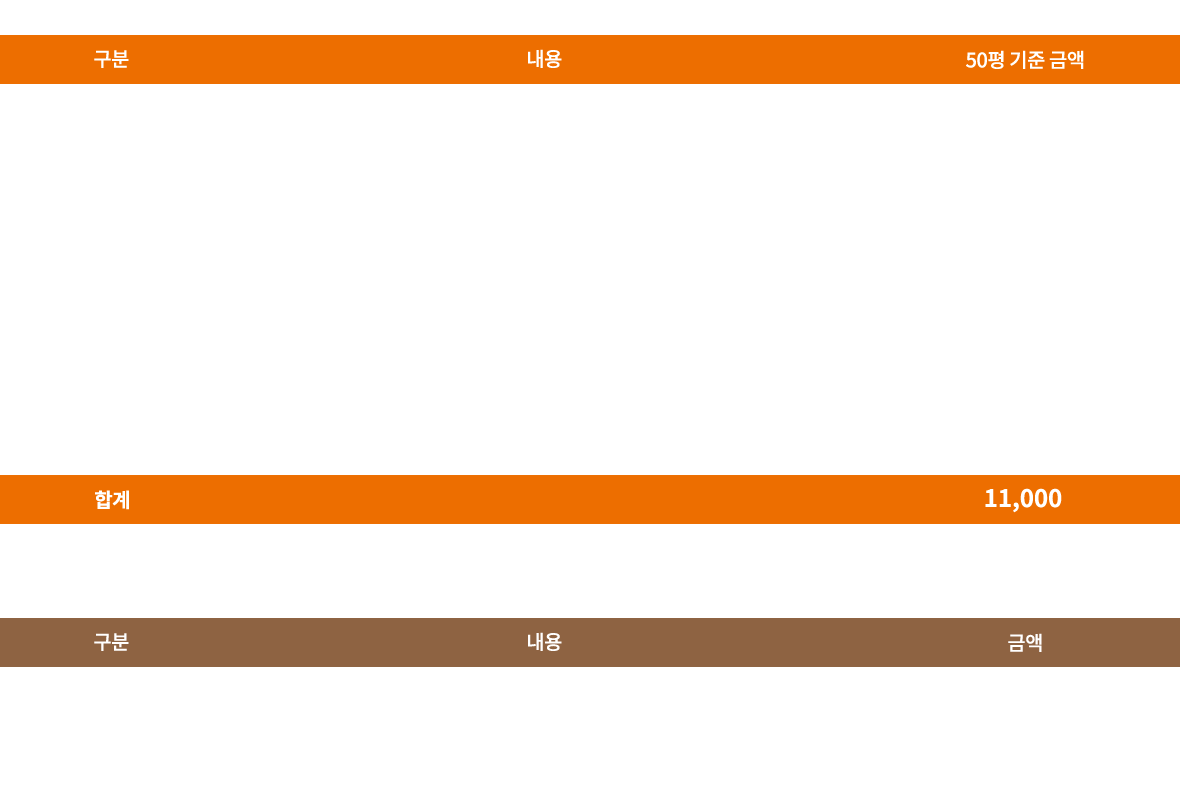
창업비용
가맹문의

매장 찾기
78개의 매장이 검색되었습니다.
-
78
- 구암앤드오션점
- 전북 군산시 궁포2로 4
-
77
- 청주하나로마트점
- 충북 청주시 상당구 무농정로 32 하나로마트 1층 푸드코트 내
-
76
- 평화점
- 전북 전주시 완산구 모악로 4678
-
75
- 삼학점
- 전북 군산시 미원로 35
-
74
- 농수산시장점
- 전북 전주시 덕진구 송천중앙로 233-13
-
73
- 청라원창점
- 인천 서구 중봉대로 394
-
72
- 진해용원점
- 경남 창원시 진해구 용원서로 29
-
71
- 서산점
- 충남 서산시 성연면 성연2로 30 1층
-
70
- 순천신대점
- 전남 순천시 해룡면 매안로 71
-
69
- 신풍점
- 전북 김제시 금성로 80
-
68
- 고창터미널점
- 전북 고창군 고창읍 보릿골로 136 1층
-
67
- 덕산점
- 충북 진천군 덕산읍 덕금로 838 1층 101호 102호
-
66
- 대학병원점
- 전북 전주시 덕진구 백제대로 686
-
65
- 남해점
- 경남 남해군 남해읍 남해대로 2744
-
64
- 남원시청점
- 전북 남원시 시청로 47
-
63
- 나운점
- 전북 군산시 하나운로 70 B동 101호(유앤미프라자)
-
62
- 김제요촌점
- 전북 김제시 중앙로 107
-
61
- 금산사점
- 전북 김제시 금산면 모악로 464 1동 4호
-
60
- 구로점
- 서울 관악구 조원로2길 48
-
59
- 봉동점
- 전북 완주군 봉동읍 봉동로 125
-
58
- 고창점
- 전북 고창군 고창읍 중앙로 319
-
57
- 부송점
- 전북 익산시 부송로 126
-
56
- 부평점
- 인천 부평구 경원대로 1422 대덕아크로존 1층
-
55
- 삼호점
- 전남 영암군 삼호읍 삼호중앙로 225
-
54
- 한서대점
- 충남 서산시 해미면 한서2길 3
-
53
- 서천점
- 충남 서천군 서천읍 사곡로 22
-
52
- 서호점
- 전북 전주시 덕진구 송천로 30
-
51
- 세종고운점
- 세종특별자치시 만남로 16
-
50
- 소룡점
- 전북 군산시 공항로 118
-
49
- 수송점
- 전북 군산시 수송안길 21
-
48
- 배산신도시점
- 전북 익산시 선화로1길 12
-
47
- 미룡점
- 전북 군산시 미제길 14
-
46
- 문화점
- 전북 군산시 문화로 17 24시간
-
45
- 문막점
- 강원특별자치도 원주시 문막읍 원문로 1800
-
44
- 목포점
- 전남 목포시 대양산단로 76
-
43
- 수송현대점
- 전북 군산시 문화로 117
-
42
- 삼천점
- 전북 전주시 완산구 용리로 20
-
41
- 모현점
- 전북 익산시 고현로 55
-
40
- 아중2호점
- 전북 전주시 덕진구 아중로 146
-
39
- 아중점(정가네)
- 전북 전주시 덕진구 건산로 259
-
38
- 만성점
- 전북 전주시 덕진구 만성남7길 4 1층 101호(만성동)
-
37
- 하가점
- 전북 전주시 덕진구 가리내10길 12-4
-
36
- 영등점
- 전북 익산시 고봉로 354
-
35
- 호성점
- 전북 전주시 덕진구 소리로 206
-
34
- 동두천점
- 경기 동두천시 장고갯로 48
-
33
- 마곡디엠시티점
- 서울 강서구 마곡동로10길 23 1층
-
32
- 오식도점
- 전북 군산시 가도로 177
-
31
- 약촌신흥점
- 전북 익산시 약촌로 51
-
30
- 부천역점
- 경기 부천시 부천로 118
-
29
- 이서혁신점
- 전북 완주군 이서면 지사제로 3
-
28
- 에코시티점
- 전북 전주시 덕진구 초포로 172
-
27
- 임실점
- 전북 임실군 임실읍 수정로 46-17 1층
-
26
- 도청점
- 전북 전주시 완산구 석산2길 17-9
-
25
- 효자직영점
- 전북 전주시 완산구 거마평로 180
-
24
- 익산동산점
- 전북 익산시 서동로 90
-
23
- 운봉점
- 전북 남원시 운봉읍 운봉로 753
-
22
- 송천점
- 전북 전주시 덕진구 사근1길 51
-
21
- 지곡점
- 전북 군산시 의료원로 40
-
20
- 남원조산점
- 전북 남원시 가방뜰길 110
-
19
- 구시장점
- 전북 군산시 대명4길 9
-
18
- 서순천점
- 전남 순천시 서면 순천로 52
-
17
- 인주점
- 충남 아산시 인주면 서해로 590
-
16
- 세종장군점(신)
- 세종특별자치시 장군면 장척로 513
-
15
- 청주율량본점
- 충북 청주시 청원구 율량로 34
-
14
- 웅천점
- 충남 보령시 웅천읍 장터중앙길 70
-
13
- 보령동대점
- 충남 보령시 작은오랏1길 23
-
12
- 인화점
- 전북 익산시 인북로4길 16
-
11
- 장성점
- 전남 장성군 장성읍 충무1길 3
-
10
- 장수군청점
- 전북 장수군 장수읍 장천로 125
-
9
- 장수점
- 전북 장수군 장계면 의암로 1065
-
8
- 장항점
- 충남 서천군 장항읍 신창서로 80
-
7
- 전주동산점
- 전북 전주시 덕진구 편운로 12
-
6
- 조촌점
- 전북 군산시 백릉로 64
-
5
- 중화산점
- 전북 전주시 완산구 화산천변2길 7
-
4
- 진안점
- 전북 진안군 진안읍 시장길 22
-
3
- 코아루점
- 전북 완주군 봉동읍 과학로 1001
-
2
- 태안점
- 충남 태안군 태안읍 서해로 2008
-
1
- 서천서면점
- 충남 서천군 서면 서인로 764 1층